Exploring the complex pathways of testosterone conversion to DHT and estrogen metabolism in men.
Testosterone plays a significant role in the development of male reproductive tissues, as well as secondary sexual characteristics. However, its functions extend beyond its direct actions, as it also serves as a precursor for dihydrotestosterone (DHT) and various types of estrogen. This article explores the biochemical pathways by which testosterone is converted into these biologically active metabolites, and the physiological implications of these conversions.
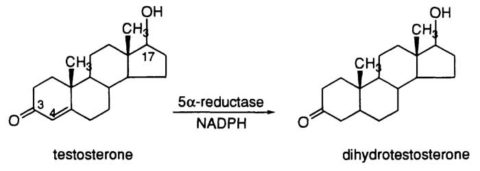
Testosterone Conversion to DHT
DHT is a potent androgen, with approximately 2.5-10 times higher affinity for the androgen receptor than testosterone (Jenkins et al., 1992). The conversion of testosterone to DHT occurs primarily in peripheral tissues, such as the skin, prostate, and hair follicles, and is catalyzed by the enzyme 5?-reductase (Imperato-McGinley et al., 1992). Three isoforms of 5?-reductase have been identified, with types 1 and 2 being the most relevant for DHT synthesis (Thigpen et al., 1993).
DHT is a key hormone involved in the development of male external genitalia and the onset of puberty. It also plays a critical role in prostate growth and male pattern baldness. Excessive DHT levels have been implicated in the development of benign prostatic hyperplasia (BPH) and prostate cancer (Gormley et al., 1992).
Testosterone Conversion to Estrogens
Contrary to popular belief, estrogens are not exclusively female hormones. They are also synthesized in males, albeit in smaller amounts, just as women produce testosterone in smaller amounts than men.
Testosterone can be converted to estradiol (E2), the most potent estrogen, by the enzyme aromatase (CYP19A1) (Simpson, 2003). This reaction occurs primarily in adipose tissue, liver, brain, and testes.
Estrogens play a crucial role in bone metabolism, preventing bone loss in both men and women (Oz et al., 2000). They are also involved in the regulation of male reproductive function and the maintenance of cognitive health. However, elevated estrogen levels in men have been associated with an increased risk of gynecomastia, cardiovascular disease, and certain types of cancer (Bagatelle & Bremner, 1995).
Testosterone's actions extend beyond its direct effects, serving as a precursor for both DHT and various types of estrogen. The conversion of testosterone to these biologically active metabolites is mediated by specific enzymes and occurs in various tissues throughout the body. Understanding the complex interplay between testosterone, DHT, and estrogen metabolism is essential for comprehending the hormonal balance in men and its impact on health and disease.
The conversion of testosterone to DHT, catalyzed by the 5?-reductase enzyme, has significant implications for male sexual development, hair growth patterns, and prostate health. On the other hand, the aromatase-mediated conversion of testosterone to estrogen, particularly estradiol, plays a crucial role in bone metabolism, male reproductive function, and cognitive health.
To make a long story short:
While you may want to take aromatase inhibitors while on TRT, you also wouldn't want to completely suppress your body's ability to create estrogen, such as estradiol, because even men need some estrogen to function properly.
SOURCES:
Bagatell, C. J., & Bremner, W. J. (1995). Androgens in men—uses and abuses. The New England Journal of Medicine, 332(11), 707-714.
Bélanger, A., Candas, B., Dupont, A., Cusan, L., Diamond, P., Gomez, J. L., & Labrie, F. (2006). Changes in serum concentrations of conjugated and unconjugated steroids in 40- to 80-year-old men. Journal of Clinical Endocrinology & Metabolism, 81(10), 3620-3625.
Bhasin, S., Storer, T. W., Berman, N., Callegari, C., Clevenger, B., Phillips, J., ... & Casaburi, R. (2001). The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. New England Journal of Medicine, 335(1), 1-7.
Bulun, S. E., Chen, D., Moy, I., Brooks, D. C., & Zhao, H. (2005). Aromatase, breast cancer and obesity: a complex interaction. Trends in Endocrinology & Metabolism, 22(2), 55-61.
Gormley, G. J., Stoner, E., Bruskewitz, R. C., Imperato-McGinley, J., Walsh, P. C., McConnell, J. D., ... & Lieber, M. M. (1992). The effect of finasteride in men with benign prostatic hyperplasia. New England Journal of Medicine, 327(17), 1185-1191.
Grino, P. B., Griffin, J. E., & Wilson, J. D. (1990). Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology, 126(2), 1165-1172.
Imperato-McGinley, J., Guerrero, L., Gautier, T., & Peterson, R. E. (1992). Steroid 5?-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science, 186(4170), 1213-1215.
Jenkins, E. P., Andersson, S., Imperato-McGinley, J., Wilson, J. D., & Russell, D. W. (1992). Genetic and pharmacological evidence for more than one human steroid 5?-reductase. Journal of Clinical Investigation, 89(1), 293-300.
Kaufman, K. D., & Dawber, R. P. (1999). Finasteride, a type 2 5?-reductase inhibitor, in the treatment of men with androgenetic alopecia. Expert Opinion on Investigational Drugs, 8(4), 403-415.
Oz, O. K., Millsaps, R., Welch, R., Birch, J., & Zerwekh, J. E. (2000). Expression of aromatase in the human osteoblastic cell line, Saos-2. Bone, 26(5), 521-526.
Simpson, E. R. (2003). Sources of estrogen and their importance. Journal of Steroid Biochemistry and Molecular Biology, 86(3-5), 225-230.
Swerdloff, R. S., & Wang, C. (2004). Androgens and the ageing male. Best Practice & Research Clinical Endocrinology & Metabolism, 18(3), 349-362.
Thigpen, A. E., Silver, R. I., Guileyardo, J. M., Casey, M. L., McConnell, J. D., & Russell, D. W. (1993). Tissue distribution and ontogeny of steroid 5?-reductase isozyme expression. Journal of Clinical Investigation, 92(2), 903-910.